Rethinking Stem Cell Transplantation in Fanconi Anemia
The field of hematopoietic stem cell transplantation has always been full of problems when it comes to treating conditions like Fanconi anemia. Traditional conditioning regimens—with their heavy reliance on genotoxic irradiation and chemotherapy drugs like busulfan—have long been associated with a host of side effects ranging from overwhelming short-term toxicities to nerve-racking long-term complications. Recent developments, however, are giving new hope that we can figure a path toward safer, more effective therapies for those suffering from this rare bone marrow failure disorder.
Anti-CD117 Therapy: A Fresh Approach Without the Genotoxic Side Effects
One promising strategy that has caught the attention of researchers and clinicians alike is the use of an anti-CD117 antibody as a conditioning agent. This method aims to target host hematopoietic stem and progenitor cells, thereby reducing the need for traditional radiation or chemotherapy. This novel antibody‐based conditioning regimen is designed to clear the bone marrow niche in a less intimidating way compared to the standard genotoxic protocols.
The concept is simple but innovative: rather than exposing patients—many of whom have DNA-repair deficiencies—to genotoxic agents that produce tangled issues, we now have a method that gently paves the way for donor cell engraftment while limiting collateral damage. This approach is not only essential for reducing tissue damage but also super important for preventing secondary complications such as infertility and secondary malignancies.
Key Benefits of Non-Genotoxic Conditioning
When we take a closer look at the finer details of the new conditioning regimen, several benefits become apparent. Here are some of the main advantages:
- Reduced Short-Term Toxicities: The antibody-based approach minimizes issues such as mucositis and organ damage, which are common with traditional treatments.
- Lower Risk of Long-Term Complications: Avoiding the use of irradiation and busulfan helps reduce the risk of secondary cancers and long-term organ toxicity.
- Enhanced Engraftment: The regimen supports prompt donor cell engraftment with rapid recovery of blood counts, contributing to successful transplantation outcomes.
- Personalized Dosing: Pharmacokinetic monitoring enables tailored dosing, ensuring that each patient receives the right amount of therapy for optimal outcomes.
These benefits underscore the importance of moving away from the old, nerve-wracking methods of conditioning toward safer alternatives that are designed to maintain high levels of efficacy while reducing risks.
Safety and Effectiveness: What the Phase 1b Trial Tells Us
A recent phase 1b trial has provided intriguing insights into the safety and efficacy of an irradiation- and busulfan-free conditioning regimen. In the trial, children with Fanconi anemia suffering from bone marrow failure were administered a single dose of an anti-CD117 antibody—briquilimab—followed by a short course of immunosuppression. The key goals of the study were to see if the approach could eliminate the need for toxic irradiation and if it would still allow donor cells to engraft successfully.
After a thorough two-year follow-up, the study outcomes were encouraging:
- Stable Engraftment: Each patient achieved robust and persistent donor chimerism. This means that the new donor cells were able to take hold and steadily replace the defective host cells.
- Minimal Toxicities: Aside from the typical mucositis—likely due to the inclusion of agents such as cyclophosphamide—the patients experienced few severe side effects. There was no evidence of veno-occlusive disease or other serious complications.
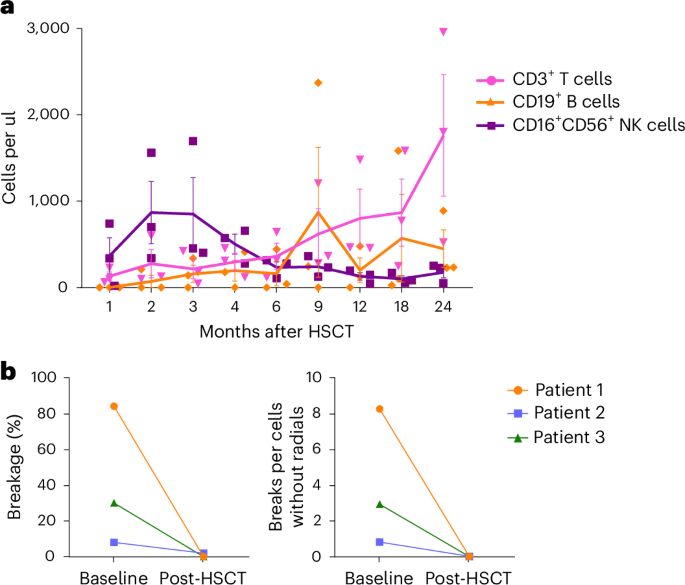
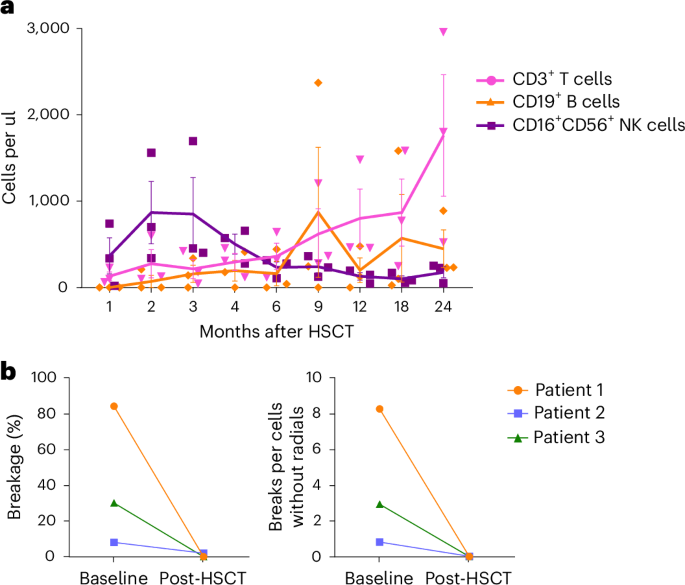
- Effective Immune Reconstitution: Beyond just blood count recovery, the patients’ immune systems reassembled effectively. This was confirmed by detailed analyses showing recovery of T cells, B cells, and natural killer cells.
By steering through the challenging parts of transplantation, the trial demonstrated that an antibody-based conditioning protocol could not only match the efficacy of traditional treatments but potentially offer a much safer alternative for patients who are particularly at risk due to their inability to repair DNA damage.
In-Depth Look at Immunosuppression and Engraftment Kinetics
One of the trickier parts of any transplant procedure is managing immunosuppression without causing additional complications. In this trial, the conditioning regimen was enhanced with a well-planned schedule that included lymphodepleting agents such as rabbit anti-thymocyte globulin (rATG), fludarabine, cyclophosphamide, and rituximab. These agents work together to reduce the host’s immune response so that donor cells can establish themselves without triggering a rejection reaction.
Detailed pharmacokinetic studies confirmed that the levels of these drugs were within the targeted ranges and that the timing of dosage was optimized according to each patient’s needs. The following table summarizes the regimen schedule along with dosing details:
| Drug | Dose | Administration Days | Purpose |
|---|---|---|---|
| Briquilimab (anti-CD117) | 0.6 mg/kg | Day -12 | Target host HSPCs clearance |
| rATG | 2.5–4 mg/kg per dose | Days -10 to -7 | Lymphodepletion to prevent rejection |
| Fludarabine | 35 mg/m2 | Days -5 to -2 | Assist in immunosuppression |
| Cyclophosphamide | 10 mg/kg | Days -5 to -2 | Further immunosuppression |
| Rituximab | 200 mg/m2 | Day -1 | Prevents EBV reactivation |
This careful combination allowed patients to not just survive but thrive post-transplantation, sidestepping some of the overwhelming side effects that have historically been part of conventional protocols.
Managing the Tricky Parts of Donor Cell Engraftment
A critical measure of success in any stem cell transplant is donor cell engraftment, which reflects the timely and complete takeover by donor cells in the patient’s bloodstream. In this trial, neutrophil engraftment was achieved within a median of just 11 days, and platelet recovery followed closely in around 13 days on average.
Rapid engraftment means that patients could return to normal life faster, with reduced reliance on transfusions and less time facing the nerve-wracking risk of infection during periods of severe immunosuppression.
Key factors contributing to this smooth and successful engraftment included:
- The Use of αβ-Depleted Grafts: By removing certain immune cells (namely, TCRαβ+ T cells and CD19+ B cells), the grafts reduced the risk of triggering graft-versus-host disease (GvHD).
- Optimized Cell Doses: Ensuring that the CD34+ cell dose was above a critical threshold helped secure robust stem cell repopulation.
- Proactive Pharmacokinetic Monitoring: Regular monitoring of drug levels helped the medical team fine-tune dosing to avoid both under- and overdose scenarios.
These measures work together to minimize the confusing bits and tangled issues that often arise during the engraftment phase. In the long run, this creates an environment where patients can enjoy lasting benefits from their transplants without the heavy baggage of treatment-related complications.
Addressing the Small Details: Immune Reconstitution and DNA Repair
One of the super important outcomes of the new conditioning regimen is the restoration of a fully functioning immune system. In Fanconi anemia, defective DNA repair mechanisms make patients particularly vulnerable to the long-term effects of genotoxic treatments. By using an antibody-based regimen, doctors are not only preserving the existing cellular architecture but also promoting a healthy recovery of the immune system.
Lab tests performed after transplant revealed encouraging results, including:
- Normalization of Blood Cells: Post-transplant, patients developed a full spectrum of donor-derived blood cells, including neutrophils, platelets, and red blood cells.
- Restored DNA Damage Resistance: Studies showed that chromosomal breakage, which is typically high in Fanconi anemia, normalized after successful transplantation.
- Rebuild of the Immune Network: The recovery of T cells, B cells, and NK cells helps to protect patients against infections and contributes to overall resilience.
These outcomes are a testament to the benefits of using non-genotoxic conditioning. They ensure that the cure does not generate its own set of complicated pieces, offering instead a balanced, long-term solution to the underlying disease pathology.
Overcoming the Overwhelming Challenges of Traditional Conditioning
For decades, the standard treatment for Fanconi anemia involved intense, DNA-damaging conditioning regimens that produced many overwhelming side effects. The traditional methods, though effective at facilitating donor cell engraftment, often left patients facing a difficult road to recovery. The side effects—ranging from severe mucositis and organ toxicity to life-long increased cancer risk—made the entire process feel off-putting for practitioners and patients alike.
The new antibody-based protocols address these challenges by removing the need for harmful irradiation and chemotherapy drugs. The approach is built around a clear understanding of the host’s vulnerabilities and is designed to sidestep the reserved power of traditional methods. Instead of wading through the nitty-gritty of DNA-damage, the focus shifts to targeted depletion of the patient’s problematic cell populations with a smart and patient-friendly strategy.
This shift represents a significant moment in the field of transplantation, one that allows both doctors and patients to work together through the strategy’s twists and turns and emerge on the other side with fewer long-term side effects and improved overall quality of life.
Fine Points of Implementing a Non-Genotoxic Transplant Protocol
Implementing any new treatment protocol is always full of problems, and the non-genotoxic approach is no exception. There are several fine points and subtle parts of the process that clinicians need to be aware of:
- Precise Timing of Drug Administration: The success of engraftment relies on the careful synchronization of antibody clearance and immune suppression. The briquilimab dose must be fully cleared before donor cell infusion, a critical factor measured meticulously through real-time pharmacokinetic studies.
- Monitoring Drug Levels Continuously: Regular blood tests are essential for ensuring that drug concentrations are kept within the optimal window. This proactive catch-and-adjust method helps to avoid any nerve-racking surprises related to drug accumulation or undue exposure.
- Handling the Challenging Immune Environment: Even though the new regime minimizes toxic exposure, the entire process of managing immune suppression still requires careful monitoring. Small distinctions—such as slight differences in dosing requirements between individuals—can have a big impact on overall outcomes.
- Supportive Care: Supportive measures, including nutritional support and pain management, remain key to addressing the nerve-wracking aspects of mucositis and other side effects that may still occur.
Looking at these points, one can appreciate the importance of every little detail in ensuring that the transition from traditional to antibody-based conditioning protocols is as smooth as possible. It is this attention to the subtle parts that promises to lead the way toward a more patient-friendly treatment paradigm.
Future Implications for Treatment of Fanconi Anemia and Other Disorders
The implications of successful non-genotoxic conditioning extend far beyond Fanconi anemia. Many bone marrow failure syndromes and other hematological disorders could benefit from protocols that avoid damaging DNA. With the reduced-intensity regimen showing promising early results, there is hope that this strategy will soon become a foundational treatment approach for patients facing a wide array of blood diseases.
If future studies continue to produce positive outcomes, we could witness a paradigm shift in the way that allogeneic hematopoietic stem cell transplantation is performed. Here are some potential future benefits:
- Expanded Patient Eligibility: Since the new regimen is far less intimidating in terms of side effects, more patients—especially those with underlying DNA repair deficiencies—may become eligible for transplant.
- Early Intervention Opportunities: With a safer conditioning method available, clinicians might be able to step in earlier, possibly before severe bone marrow failure sets in, which could improve overall prognosis.
- Broader Applications: The success of an antibody-based protocol could inspire similar approaches in treating other conditions where traditional conditioning provokes more harm than benefit.
- Reduced Long-Term Healthcare Costs: Minimizing long-term side effects such as secondary cancers or chronic organ damage can lead to significant reductions in healthcare expenditure and improve the overall quality of life for patients.
This future outlook is truly inspiring and suggests that with continued research and clinical fine-tuning, the tricky parts of using genotoxic agents can be finally left behind in favor of far more patient-friendly options.
Personalizing Care Through Detailed Pharmacokinetic Monitoring
Another key aspect of the new approach is its commitment to personalized care through detailed pharmacokinetic (PK) monitoring. By collecting and analyzing blood samples at multiple time points after drug administration, clinicians are able to adjust dosing in a finely tuned manner that suits the unique metabolism of each patient.
This thorough process is especially critical when dealing with immune suppression and engraftment, as there can be slight differences in how patients process drugs like rATG and fludarabine. Some highlights include:
- Real-Time Adjustments: Real-time PK data allow doctors to make immediate adjustments to dosing schedules, ensuring that drug levels stay within a safe and effective range.
- Minimized Variability: By addressing the fine details of each patient’s drug metabolism, variability in therapeutic outcomes can be minimized.
- Enhanced Patient Safety: Continuous monitoring provides a safety net, catching any deviations before they lead to problematic side effects or compromise engraftment.
This personalized and detailed monitoring process could serve as a model for future treatments and underscores the growing trend in modern medicine toward individualized treatment strategies—a trend that is essential when managing conditions full of tangled issues like Fanconi anemia.
Managing Post-Transplant Challenges With a New Mindset
Even with a promising new regimen in place, the post-transplant phase continues to require careful management. While acute graft-versus-host disease was largely avoided in the trial, some patients did experience moderate chronic symptoms or viral reactivations. These incidents remind us that no treatment approach is absolutely free from challenges.
However, the observed issues were typically managed with supportive care and targeted therapies, and did not detract from the overall positive outcomes of the procedure. Key strategies for managing these post-transplant challenges include:
- Vigilant Monitoring: Continuous follow-up visits and laboratory tests help detect any early signs of trouble so that interventions can be made promptly.
- Responsive Immunomodulation: For those instances where mild to moderate immune reactions occur, clinicians can introduce brief courses of immunosuppressants to steer through the rough patches.
- Proactive Antiviral Therapy: Since viral reactivations can sometimes occur post-transplant, the use of prophylactic or early antiviral treatment has proven beneficial.
- Patient Education: Empowering patients with knowledge about the signs and symptoms of complications helps ensure that they seek timely help—turning potentially overwhelming situations into manageable ones.
These management strategies illustrate the importance of a comprehensive care approach that goes beyond the transplant itself, addressing even the confusing bits that might occur after the donor cells have taken root.
Looking Ahead: The Road to a Fully Non-Genotoxic Transplantation Regimen
While the current results are highly promising, the journey toward a fully non-genotoxic transplantation regimen is still underway. Future research must aim to further refine the conditioning process—ideally eliminating any residual exposure to DNA-damaging agents. The ultimate goal is to create a protocol that can be applied so early in the disease course that patients might even avoid the progressive bone marrow failure that has historically defined Fanconi anemia.
Future research avenues and improvements could involve:
- Fine-Tuning the Regimen: Researchers may experiment with removing even low-dose chemotherapeutics like cyclophosphamide or fludarabine, provided that alternative methods can still secure successful engraftment without rejection.
- Expanding Patient Populations: As the technique becomes more refined, it could be introduced to a broader range of patients—not only those with Fanconi anemia but also others with severe hematological disorders who may benefit from this less damaging approach.
- Long-Term Outcome Studies: Extended follow-up over decades will be necessary to confirm that the reduction in genotoxic exposure translates to better long-term survival and quality of life.
- Integration with Other Emerging Therapies: Combining non-genotoxic conditioning with other innovative therapies, such as gene editing and gene therapy, could further enhance personalized care options and long-term outcomes.
These future directions are exciting because they show that even the most intimidating aspects of traditional conditioning can eventually be replaced by smarter, more flexible strategies that respect the unique needs of patients with DNA-repair deficiencies.
Concluding Thoughts: A New Era for Hematopoietic Transplantation
The developments in non-genotoxic, antibody-based conditioning paradigms represent a significant turning point in the treatment of Fanconi anemia. By relying on anti-CD117 antibodies to target host stem cells, clinicians are now able to steer through the previously nerve-wracking drug regimens that have long been a necessary evil. Instead of managing a landscape loaded with issues related to irradiation or high-dose chemotherapy, this method offers a way to safely and effectively prepare patients for life-saving transplantation.
It is important, however, to remain cautiously optimistic. The early-phase study involved only a small group of patients, and while the results are highly encouraging—showing rapid engraftment, low toxicity, and effective immune reconstitution—additional research will be needed. Larger, long-term studies are required to confirm that these benefits persist over the years and translate into lower rates of secondary malignancies and other complications.
Nonetheless, the current evidence indicates that we have found a promising alternative in an area once riddled with tensions and overwhelming risks. The path through this innovative treatment method is one of carefully measuring drug levels, personalizing care, and protecting vulnerable cells from the damaging side effects of traditional approaches.
As we take a closer look at this evolving paradigm, it becomes clear that the future of hematopoietic stem cell transplantation might not be built on the old, heavy-handed methods of irradiation and busulfan-based chemotherapy. Instead, the future could very well be defined by precision, reduced toxicity, and a commitment to preserving the integrity of the patient’s own biological mechanisms.
In conclusion, the journey to a fully non-genotoxic conditioning regimen is filled with challenging twists and turns, but each step brings us closer to a future where life-saving transplants can be performed with less risk, better outcomes, and ultimately a higher quality of life for patients with Fanconi anemia and other blood disorders. By embracing innovative techniques like antibody-based conditioning, the medical community is not only offering hope to those who suffer from these conditions but is also charting a course toward truly personalized and less harmful treatment strategies.
The road ahead might still be full of tangled issues and subtle details that require ongoing exploration and improvement through research, but the progress made thus far is both commendable and promising. In managing the confusing bits of traditional conditioning protocols, this new approach could offer a safer, more approachable treatment that helps both patients and clinicians find their way through one of the most challenging aspects of modern medicine.
Ultimately, the impact of these advancements goes beyond Fanconi anemia alone. They signal a broader shift toward treatments that respect the delicate balance of the human body while offering robust, life-saving interventions—a shift that is needed in an era where personalized and targeted therapies are becoming the norm.
Originally Post From https://www.nature.com/articles/s41591-025-03817-1
Read more about this topic at
A new step toward non-genotoxic conditioning prior to …
Non-genotoxic conditioning facilitates hematopoietic stem …