Introduction: Navigating the Changing Landscape of Prostate Cancer Treatments
The current era in cancer research is characterized by exciting new findings and fresh perspectives on how to tackle stubborn diseases. In recent studies, scientists have turned their attention to a hormone receptor, TRβ, which may hold the key to slowing the growth of aggressive prostate cancer. This opinion editorial offers a deep dive into the research, examines its implications, and reflects on the potential of targeting TRβ as part of a holistic strategy to develop the next generation of cancer therapies and analgesics.
Prostate cancer remains one of the most common cancers among men worldwide, and treating its advanced forms has proven to be one of the more nerve-racking challenges in oncology. With early-stage disease often managed by lowering testosterone levels, many men experience treatment resistance over time. This article unpacks the recent discovery that connects tangled issues in thyroid hormone signaling with cancer growth and offers hope for new treatment avenues, including the promise of precision therapeutic approaches.
Investigating the Role of Thyroid Hormone Receptor Beta (TRβ) in Cancer Growth
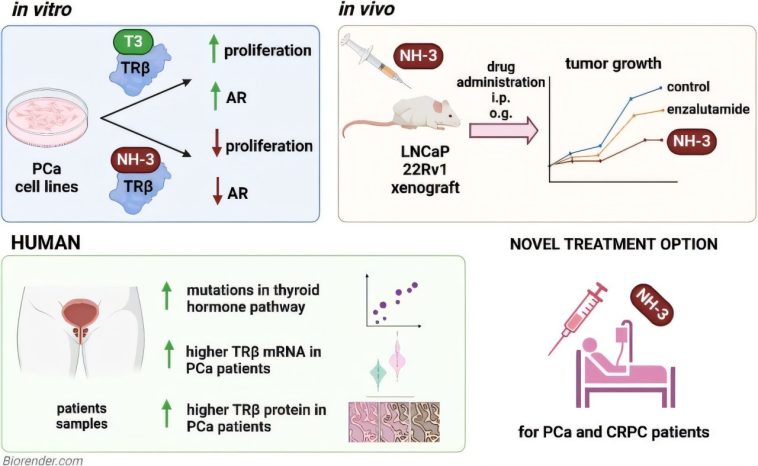
Recent international research undertaken by teams at Umeå University and the Medical University of Vienna has shed new light on how a receptor known as TRβ plays a critical role in the evolution of prostate tumors. This receptor binds to the thyroid hormone triiodothyronine (T3), and when T3 activates it, studies have shown a considerable increase in the proliferation of prostate cancer cells.
The findings indicate that TRβ may be a driving force behind the growth of cancer. When TRβ is inhibited, however, using research compounds like NH-3, the expansion of tumor cells is significantly curtailed. This discovery is a classic example of how addressing one of the subtle details in the signaling pathways can lead to potential breakthroughs in treatment.
Understanding TRβ’s Mechanisms in Prostate Cancer
At the core of this research is the mechanism by which TRβ influences the behavior of prostate cancer cells. Scientific experiments have confirmed the following points:
- T3, the thyroid hormone, binds to TRβ and initiates a cascade of signals that encourages cell growth.
- Activation of these pathways leads to a surge in prostate cancer cell numbers.
- Blocking TRβ with a research compound, NH-3, results in a marked reduction in tumor cell proliferation.
- Therefore, TRβ stands out as a promising target for developing future drugs that might stop or slow down the progression of aggressive prostate cancer.
This series of experiments suggests that even though hormone-based mechanisms often involve complicated pieces of biochemical processes, researchers can unveil neat strategies by focusing on small distinctions, such as how specific receptors interact with hormones.
Assessing Animal Studies: Overcoming the Intimidating Challenges of Castration-Resistant Prostate Cancer
One of the most compelling features of the research on TRβ is the follow-up validation through animal studies. In experiments with mice, tumors treated with the TRβ blocker NH-3 either stayed smaller or showed slower progression, particularly in models simulating castration-resistant prostate cancer. This form of prostate cancer continues to progress despite conventional treatment strategies, making these findings especially promising.
Animal studies provide a critical platform to understand the complex pieces of cancer evolution in a living organism. Often, these experiments highlight the twists and turns of biochemical signals that are hidden in cell cultures, offering a more realistic glimpse into how treatments might perform in humans.
When it comes to issues as intimidating as castration-resistant prostate cancer, the following points deserve close attention:
- Modeling the Disease: Mouse models have allowed researchers to mimic the human condition of treatment resistance, thereby unveiling hidden complexities in tumor progression.
- The Androgen Receptor Link: Blocking TRβ was observed to eliminate an essential signal – the androgen receptor signal – which is known to be a key driver in prostate cancer development.
- Implications for Human Treatment: Though the studies are preclinical, the encouraging results open the door for future clinical trials, aimed at curbing the aggressive growth seen in many cases of prostate cancer.
From Patient Data to Precision Medicine: The Subtle Details of Thyroid Signaling in Prostate Cancer
Beyond animal studies, robust evidence from patient data plays an essential role in shaping the future of cancer treatments. In this case, researchers have observed that tumor samples from prostate cancer patients often exhibit elevated levels of TRβ compared to healthy tissue. Genetic analyses further confirm that mutations affecting thyroid signaling pathways are common among patients. This discovery is critical as it underscores the importance of developing therapies that are super important for targeting these specific mutations.
The detailed examination of patient tissue samples provides a foundation for precision medicine. By focusing on the fine points of thyroid hormone receptor levels, clinicians may be able to design treatment plans that are more targeted and customized to the individual needs of patients. A few highlights include:
- Elevated TRβ Expression: The discovery that TRβ levels are significantly higher in tumor tissues than in normal tissues suggests that thyroid signaling plays a direct role in cancer progression.
- Genetic Mutations: Patients often display specific mutations in the thyroid hormone pathways, which could potentially serve as biomarkers for aggressive forms of cancer.
- Future Therapeutic Strategies: Targeting TRβ alongside or in combination with existing treatments might pave the way for innovative strategies that break down the tangled issues in treatment-resistant prostate cancer.
Potential for Combining TRβ Blockade with Current Therapeutic Practices
While the notion of targeting TRβ is compelling, it must be considered within the larger context of existing prostate cancer treatments. Presently, early-stage prostate cancer is often managed through hormonal therapies that reduce testosterone levels, yet this approach eventually loses its effectiveness in many patients. Hence, the possibility of combining TRβ inhibition with current hormonal therapies represents a promising multi-pronged strategy.
Here are some considerations in this blended approach:
- Dual Targeting: A combination strategy could involve simultaneously blocking TRβ and reducing testosterone-driven signals, tackling the cancer cells via two distinct mechanisms.
- Minimizing Side Effects: By focusing on multiple pathways, it might be possible to administer lower doses of each agent, thereby reducing the risk of side effects that can sometimes be overwhelming.
- Personalised Treatment Plans: As research on TRβ evolves, it could become super important to create personalized patient profiles based on the expression of thyroid hormone receptors and related genetic markers.
Overall, integrating TRβ blockade with standard therapies could represent an evolution in clinical practice, aiming to manage the tricky parts of cellular signaling and overcome resistance mechanisms.
Exploring the Broader Impact on Drug Discovery and Analgesic Development
The potential ramifications of this research extend far beyond prostate cancer alone. The methods and discoveries involved in targeting TRβ could also influence the search for next-generation analgesics – a field that has historically grappled with off-putting side effects and inconsistent efficacy.
In many early drug discovery studies, researchers have encountered various tangled issues when it comes to developing analgesics that not only relieve pain but also maintain a good safety profile. By better understanding pain pathways and delving into the precise mechanisms at play, scientists are paving the way for more targeted drugs that are less likely to cause adverse effects.
Key Messages for the Future of Analgesic Research
There are several fine points that highlight why current advances in cancer research may also lead to improvements in how we study pain:
- Precision Peptide Applications: Innovations in precision peptide research, which are closely related to methods used in blocking receptors like TRβ, could redefine how chronic and cancer-related pain is managed.
- Data-Driven Systems: By employing robust data-driven systems, researchers can manage complicated pieces of biochemical data, turning them into actionable insights for drug discovery.
- Improved Reproducibility: Enhancing reproducibility through standardizing assays and protocols is key to scaling up production of novel therapeutics that have clear clinical applications.
- Hurdle Reduction: Insights into biomarkers not only address small distinctions in patient responses but also help to reduce setbacks in translational research.
Improving our understanding of these subtle details is essential for conquering the nerve-wracking complexities involved in both cancer therapy and pain management. This research, therefore, offers a dual promise – paving the way for new cancer treatments while also informing the development of safer, more effective analgesics.
Potential Challenges and Considerations in Developing TRβ-Targeted Therapies
While the emerging evidence is highly promising, it is important to approach the implementation of TRβ-targeted treatments with caution. Cancer biology is rarely straightforward, and the path to a successful drug is laden with confusing bits that require careful analysis.
One of the key challenges is balancing the need to inhibit TRβ activity against the risk of disturbing thyroid hormone homeostasis. As research lead Lukas Kenner notes, it is a balancing act not to alter the hormonal equilibrium in the thyroid gland more than necessary. This clearly illustrates the off-putting tension that exists in the drug development process, especially when therapies have the potential to cause systemic effects.
Some of the major concerns include:
- Hormonal Balance: Any treatment that manipulates thyroid hormone signaling must be finely tuned to avoid unintended consequences on overall endocrine function.
- Variability Among Patients: Differences in genetic make-up mean that not every patient will benefit equally from TRβ inhibition. Personalized medicine approaches are therefore essential.
- Combination Therapy Risks: While combining TRβ inhibitors with other hormonal treatments may enhance efficacy, it also opens the door to potential drug-drug interactions that must be meticulously managed.
- Long-Term Safety: As with any new treatment strategy, long-term clinical trials will be essential to ascertain the safety profile of compounds like NH-3 in the context of chronic therapy.
These considerations reinforce the need for continued research, rigorous clinical testing, and a cautious, yet optimistic, approach to developing therapies that address the tricky parts of prostate cancer treatment.
Integrating Insights from Cancer Drug Discovery into Broader Medical Practices
The recent advancements in targeting TRβ not only offer new hope for prostate cancer patients but also exemplify a broader shift in how we approach drug discovery today. The traditional one-size-fits-all methods are increasingly being replaced by more nuanced, precision-based strategies that account for the little twists and subtle details unique to each patient’s condition.
This transformation is echoed in several key aspects of modern medicine:
- Data-Driven Decisions: Enhanced analytical tools allow researchers to sift through loads of data, improving decision-making speed and accuracy in the drug development process.
- Biomarker-Led Approaches: Identifying and validating biomarkers, such as elevated TRβ levels, provides a roadmap for personalized therapeutic strategies.
- Collaborative Research Efforts: International collaborations, like those between Umeå University and the Medical University of Vienna, demonstrate that pooling expertise across institutions is critical in solving the tangled issues associated with cancer.
Adopting these innovative methods helps to find your way through the maze of cancer biology and accelerates the progress of drug discovery—not only for prostate cancer but for a variety of challenging diseases. The ripple effect of such innovations will likely influence other areas of medicine, including alternative therapeutic methods and even nutritional strategies aimed at reducing cancer risks.
The Role of Biotechnology and Analytical Tools in Advancing Treatment Options
Technological advances have played a super important role in refining research methods and accelerating the pace of new discoveries. In this context, the use of advanced imaging techniques, omics analyses, and high-throughput screening methods has allowed scientists to figure a path through the tangled issues of prostate cancer treatment.
Modern biotechnology tools help researchers to:
- Visualize Cellular Pathways: Advanced imaging techniques provide a real-time glimpse into how cancer cells respond to hormone signals, offering critical clues about the effects of TRβ activation or inhibition.
- Decipher Genetic Information: Omics analyses, such as genomics and proteomics, have unraveled the genetic and protein-level changes that accompany cancer development, highlighting the small distinctions that differentiate aggressive tumors from less harmful ones.
- Develop Predictive Models: Computer-based models and bioinformatics have improved our ability to predict the impact of novel therapeutics, thus reducing the risk associated with new treatment combinations.
- Enhance Reproducibility: Standardizing assays across research networks ensures that findings are reproducible and that the intricate pieces of cellular behavior can be understood and manipulated for therapeutic benefit.
These technological advancements not only support targeted drug discovery but also help in optimizing the overall process of clinical trials and regulatory approval. As a result, patients might see more rapid improvements in treatment options, with therapies carefully optimized to address both the immediate and long-term challenges of cancer management.
Implications for Future Research and Clinical Applications
Targeting TRβ opens up several promising avenues for future research. While the current studies are largely preclinical, the next phase will undoubtedly involve clinical trials aimed at assessing the efficacy and safety of TRβ inhibitors in patients. Such trials will help to address the slight differences in individual responses based on genetics, lifestyle, and environmental factors.
In addition, future research could explore:
- Combination Strategies: Testing TRβ inhibitors alongside current standard-of-care treatments in controlled clinical environments to evaluate synergistic effects.
- Broader Molecular Profiling: Expanding genetic and proteomic studies to uncover additional biomarkers that might predict response to TRβ-targeted therapies.
- Long-Term Outcomes: Gathering data on the long-term safety and effectiveness of these new treatments is crucial, especially given the potential side effects associated with altering hormonal pathways.
Clinical researchers, pharmaceutical companies, and funding agencies will need to work together to ensure that these promising leads are translated from the laboratory bench to the patient’s bedside. It is a collective effort that demands the kind of partnerships that can tackle not only the immediate hurdles but also the tangled issues that lie further down the road in cancer therapy development.
Charting a Course Through Tricky Parts and Tangled Issues in Oncology
The journey from bench research to bedside application is often riddled with tension and evolving challenges. As we rethink traditional methods of cancer therapy, it is critical to remember that every breakthrough comes with its own set of challenging pieces. In the context of prostate cancer, the discovery of TRβ’s role is not just an isolated finding; it is part of a larger trend towards precision medicine that seeks to address both the obvious and subtle factors involved in disease progression.
Several key takeaways that could drive the next decade of research include:
| Key Aspect | Observations |
|---|---|
| Hormone Signaling | TRβ activation intensifies prostate cancer cell growth when bound to T3. |
| Research Compounds | NH-3 shows promise in inhibiting TRβ, reducing tumor proliferation in laboratory models. |
| Animal Model Evidence | Mouse studies signal that blocking TRβ may slow down castration-resistant tumor progression. |
| Patient Data | Elevated levels of TRβ in tumor tissues underscore its potential as a biomarker and therapeutic target. |
| Combination Approaches | Integrating TRβ inhibitors with existing treatments could lead to more effective and personalized therapy strategies. |
This table encapsulates the fine shades and hidden complexities inherent in oncological research today. By understanding and addressing these details, the medical community is better positioned to transform challenges into practical, effective treatment options that empower patients in the fight against cancer.
Final Thoughts: Embracing a Future of Precision Oncology and Targeted Therapies
The road to developing revolutionary cancer treatments is loaded with both promise and tricky parts. The research on TRβ provides a hopeful glimpse into what may be a turning point for patients suffering from aggressive prostate cancer. As we take a closer look at the evidence, we find that blocking a single receptor could influence a cascade of signals central to tumor growth. This creates an exciting opportunity not only for oncology specialists but also for those involved in the broader field of drug discovery and analgesic research.
It is important for clinicians and researchers alike to remain level-headed as they work through the complicated pieces of cancer treatment. Embracing innovative methodologies, such as precision medicine and data-driven analysis, can help steer through the overwhelming challenges posed by treatment-resistant cancers. At the same time, patient safety and hormonal balance must remain at the forefront of any new therapeutic strategy.
In conclusion, while the study of TRβ in prostate cancer is still in its early stages, its implications are far-reaching. The possibility of integrating targeted TRβ blockade with existing therapeutic regimens represents a super important shift towards more individualized and effective treatments. If subsequent research and clinical trials continue to support these initial findings, we may be on the cusp of a significant breakthrough—not only in oncology but also in the broader arena of pharmaceutical sciences. As the medical community continues to dig into the fine points of hormone receptor signaling and precision drug discovery, patients can look forward to a future filled with hope, innovation, and more personalized care.
Ultimately, this line of research reinforces the idea that even in the face of intimidating challenges and tangled issues, there is always room for improvement and innovation. By carefully balancing the potential risks and rewards, healthcare professionals can build on the current momentum to create therapeutic solutions that meet the evolving needs of patients worldwide. Every small victory in understanding the neurobiology of cancer, every incremental step in improving clinical trials, moves us closer to an era where targeted therapies are not just a possibility, but a reality for everyone in need.
As health systems around the globe continue to adapt to the ever-changing environment of modern medicine, it is clear that the integration of advanced biotechnology, rigorous data analysis, and innovative research initiatives will form the cornerstone of future therapeutic developments. Whether through TRβ inhibition or any number of other promising strategies, the pursuit of safer, more effective cancer treatments remains a collective goal—one that blends scientific ingenuity with compassionate clinical care.
The insights provided by studies focusing on the thyroid hormone receptor open up myriad opportunities for synergistic approaches in cancer research. From enhancing the precision of drug delivery mechanisms to devising new protocols that combine traditional and experimental treatment methods, the potential applications are as vast as they are promising. This road, though filled with nerve-racking moments and intricate twists and turns, is one that holds the promise of a brighter future for patients who have long battled aggressive cancers.
In drawing these threads together, it becomes clear that the pursuit of better cancer therapies is a dynamic and ongoing process—one that calls for collaboration, adaptability, and an unwavering commitment to patient care. As new research builds upon established knowledge, every discovery, every adjusted approach, contributes to a collective effort to conquer cancer and alleviate the physical and emotional pain it causes.
Looking ahead, it is our hope that ongoing partnerships between leading research institutions, regulatory bodies, and clinical practitioners will continue to pave the way for treatments that are tailored, targeted, and ultimately transformative. By pulling together the collective expertise in molecular biology, pharmacology, and clinical medicine, we are better equipped to make headway through those confusing bits of pathological signaling. This integrated approach is more than just an academic exercise—it is a practical, hands-on strategy to bring relief to those who need it most.
As we figure a path through the complexities of hormone-influenced cancer biology, the story of TRβ stands out as a beacon of possibility. It challenges us to rethink existing paradigms, encourages us to embrace innovative ideas, and reminds us that even in the midst of daunting challenges, progress is achievable when science and compassion work hand in hand.
Originally Post From https://www.drugtargetreview.com/news/190417/tr%CE%B2-receptor-could-be-key-to-slowing-prostate-cancer/
Read more about this topic at
Targeting Thyroid-Stimulating Hormone Receptor
Targeting Thyroid-Stimulating Hormone Receptor